Steps for Class III medical devices compliance
- Classification: ensure the device is a Class III medical device.
- Choose Conformity Assessment Route: refer the flow chart below.
- Compile the Technical File.
- Obtain certification from a Notified Body
- Declaration of Conformity.
- Appoint an Authorised Representative. (Hold the Tech Files for inspection by the Competent Authority)
- Vigilance and Post Market Surveillance. (affix CE marking & market the products)
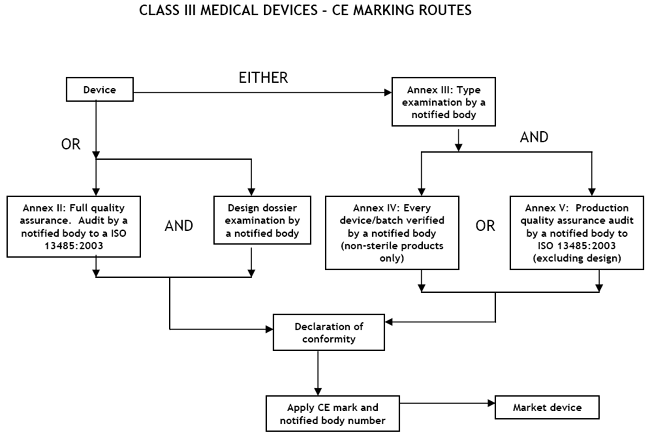
Class III Medical Devices: Conformity Assessment Routes
The conformity assessment routes for Class III Medical Devices
In the case of devices falling within Class III, other than devices which are custom-made or intended for clinical investigations, the manufacturer shall, in order to affix the CE marking, either:- follow the procedure relating to the EC declaration of conformity set out in Annex II (full quality assurance);
or - follow the procedure relating to the EC type-examination set out in Annex III, coupled with:
- (i) the procedure relating to the EC verification set out in Annex IV;
or - (ii) the procedure relating to the EC declaration of conformity set out in Annex V (production quality assurance).
- (i) the procedure relating to the EC verification set out in Annex IV;
There are two routes:
- a Notified Body must carry out either an Annex II audit of the full quality assurance system (ISO 13485:2003),plus that the manufacturer must submit the design dossier to the Notified Body for approval under Annex II, or
- a type-examination (Annex III) plus one of the two options given here:
- Examination and testing of each product or homogenous batch of products (Annex IV); or
- Audit of the production quality assurance system (Annex V:) ISO 13485:2003 (excluding Design)

No comments:
Post a Comment
Please feel free to contact or comment the article